An Analysis of Patient Safety Risks in Dental 3D Printing Post-Processing
Executive Summary
The proliferation of in-office 3D printing offers dental and orthodontic practices unprecedented agility. However, this power comes with the significant responsibility of medical device manufacturing — a reality for which many practices are unprepared. This paper addresses the critical patient safety risks stemming from inadequately controlled post-processing of 3D-printed appliances and production aids such as dental models.
The core of the issue lies with the photopolymer resins used in printing. These materials are cytotoxic in their uncured state. Think of it like dish soap. If you don’t rinse it off completely, residue remains. In 3D printing, that residue isn’t just annoying — it’s toxic. Without a rigorously validated post-processing workflow to wash and cure parts completely, residual toxic monomers can remain. This directly compromises the biocompatibility of the final appliance, whether through direct printing or through transfer from a contaminated production model. The term “biocompatible” is not a property of the resin itself, but of the final, correctly processed part.
This paper synthesizes internal investigations with peer-reviewed external research to demonstrate how seemingly minor process deviations lead to cytotoxic failures and dimensional inaccuracies. It outlines the necessity of a robust Quality Management System (QMS), including process validation (IQ, OQ, PQ, which stand for Installation Qualification, Operational Qualification, and Performance Qualification) and statistical process control (SPC), to ensure consistent and safe outcomes. Finally, it provides a balanced evaluation of when in-office printing may be appropriate versus when partnering with a dedicated, compliant manufacturing facility is the safer and more financially sound strategic decision.
Clinicians must recognize that with the benefits of 3D printing come responsibilities best managed through rigorous validation or trusted manufacturing partnerships.
Introduction: The Promise and Peril of In-Office 3D Printing
The integration of 3D printing into the modern dental practice offers compelling advantages, from rapid production of patient-specific models to the potential for creating in-house surgical guides and appliances. As the technology becomes more accessible, many clinicians are bringing this manufacturing capability into their offices.
However, the printing process itself is only the first step. The subsequent post-processing—the washing and curing of the printed object—is a critical manufacturing stage that determines the final product’s safety and efficacy. Photopolymer resins used in 3D printing are, in their uncured state, cytotoxic due to the presence of unreacted monomers [1, 2]. When post-processing is incomplete, these toxic leachables can remain.
This presents two primary avenues for patient harm. First, when an appliance is fabricated on a contaminated dental model, these leachables can transfer to the final device. Second, the risk is even more direct with “biocompatible” resins intended for printing final appliances. A critical misunderstanding is that these resins are inherently safe. In reality, biocompatibility applies only to the finished part after it has been perfectly washed and fully cured according to a validated process [3].
Understanding Cytotoxicity and Its Measurement
To appreciate the risk, it is essential to understand the method used to measure it.
What is Cytotoxicity Testing?
Cytotoxicity testing is a standard biological evaluation used to determine if a material will damage or kill living cells. For medical devices, the most common test is the ISO 10993-5 Elution Method. In this test, a device is soaked in a liquid medium. This liquid, or “eluate,” which now contains any leached chemicals, is placed in contact with a layer of living cells. After 24 – 72 hours (depending on cumulative patient contact duration), the cells are microscopically examined for damage or death (lysis).
The Grading Scale The results are scored on a 0 to 4 reactivity grade. A higher grade indicates a greater toxic effect.
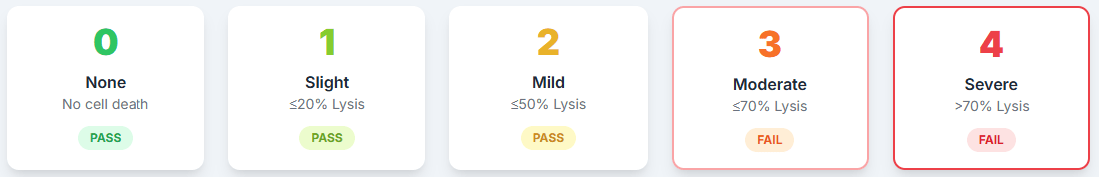
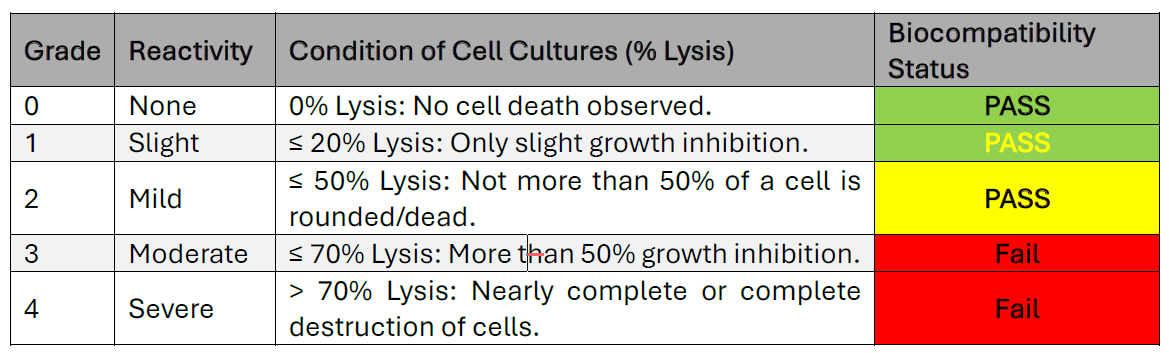
Cytotoxicity tells us how harmful a material is to living cells. In 3D printing, leftover resin that isn’t properly washed or cured can leach into the mouth and kill or damage cells. A grade of 3 or 4 means nearly 100% cell death — it’s not just risky, it’s biologically destructive.
According to ISO standards, a grade of 2 or lower is considered acceptable. However, a result of grade 2, while technically passing, indicates a process with very little margin for error. Minor, uncontrolled process shifts could easily push a device from a grade 2 (mild) to a grade 3 (moderate), resulting in a failed, non-biocompatible product.
Case Study: Four Lessons in Process Control
A commitment to patient safety requires a deep understanding of the entire manufacturing process, which can only be gained through rigorous validation. The following examples from internal quality system investigations at Specialty Appliances (SA) illustrate distinct but interconnected lessons on the critical nature of post-processing. These internal tests were designed to identify and mitigate risks before they could lead to patient harm.
Lesson 1: A Toxic Appliance Can Originate from a Contaminated Tool.
A baseline cytotoxicity test on a Hawley retainer unexpectedly yielded a Grade 4 (Severe) result, indicating 100% cell death [4]. A root cause investigation of this internal test sample revealed the toxicity originated from the 3D-printed dental model used as a production aid. This proved that an uncontrolled workflow for a tool could result in the transfer of cytotoxic material to a final device. After the workflow was validated, an identical retainer passed with a perfect Grade 0 (None) cytotoxicity score [5].

Lesson 2: Safety Margins Must Be Scientifically Proven, Not Assumed.
To understand the safety limits of the washing process, a formal Operational Qualification (OQ) was performed. This study intentionally tested “worst-case” conditions. When test models were washed with insufficient agitation speed, they failed cytotoxicity testing with a Grade 4 (Severe) result [6]. This scientifically proved that agitation is a critical-to-safety parameter. This aligns with external systematic reviews that identify post-processing techniques as a critical factor influencing biocompatibility outcomes [1, 2].
Lesson 3: “Simple” Setups Can Have Critical Failures.
During a separate validation, an Installation Qualification (IQ) of an orbital shaker revealed it had been installed on an uneven surface. Performance testing showed its actual output was only 172 RPM, despite being set to 180 RPM [7]. Knowing from Lesson 2 that agitation speed is critical, it became clear how an improper installation could inadvertently drop performance below the validated safety threshold.
Lesson 4: Manufacturer “Validation” is a Guideline, Not a Guarantee.
A manufacturer’s validated workflow provides an essential starting point, but it does not guarantee safety in a different facility. Site-specific validation is required to account for facility-level variables. This is supported by external research, which concludes that due to the heterogeneity of materials and methods, it is not possible to identify an ideal, universal post-processing protocol, reinforcing the need for process validation beyond simply following a manufacturer’s standard instructions [2]. Any deviation—such as using a different washer or alcohol purity—requires full re-validation to ensure patient safety.
These four lessons create a comprehensive picture. They show that safety depends on controlling the tools used for fabrication (Lesson 1), scientifically proving process limits (Lesson 2), ensuring even simple equipment is installed and functioning correctly (Lesson 3), and rigorously re-validating any new material or process, regardless of the manufacturer’s claims (Lesson 4). These lessons make one thing clear: in 3D printing, safety and consistency are engineered outcomes—not assumed ones.
From Validation to Production: Ensuring Consistent Safety
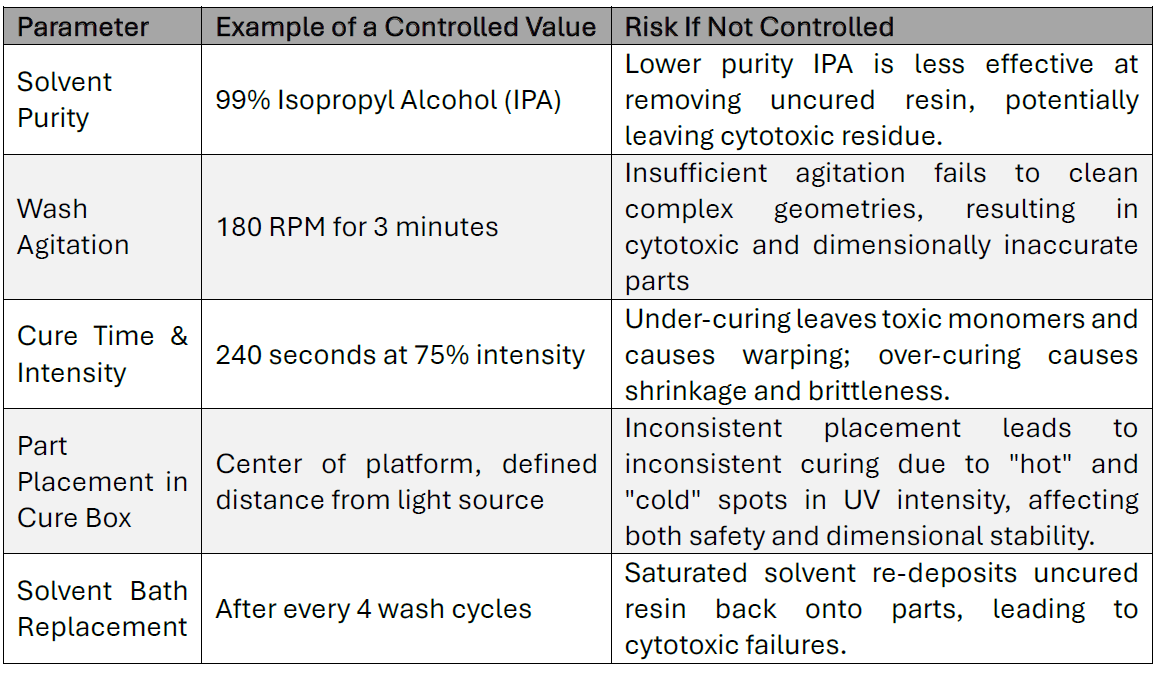
Process validation proves a workflow can produce a safe part. Maintaining that safety level in day-to-day production requires ongoing process control. The following table illustrates some of the critical parameters that must be defined and controlled, and the risks associated with deviation.
Beyond Safety: The Impact of Process Control on Dimensional Accuracy and Fit
Improper post-processing compromises more than just biocompatibility; it also dictates the final fit and accuracy of an appliance. This is supported by external research showing that different post-processing protocols significantly impact the final physical properties of a device [8]. Deviations of even a fraction of a millimeter, easily introduced by process variables, can result in a clinically unacceptable fit.
The Technical Problem: A Process of Hidden Variables
Achieving dimensional accuracy requires controlling numerous variables. Internal studies at Specialty Appliances, corroborated by external research, confirm that small changes have a significant impact:
• Improper Curing: The curing process is a “sweet spot.” Under-curing leaves the polymer network unstable, leading to warping. This unstable polymer network also leaves residual, cytotoxic unreacted monomers, directly compromising biocompatibility. Over-curing can cause excessive shrinkage. Both of which result in an inaccurate part.
• Solvent Type: Solvents used for washing can cause the resin to swell. Different solvents or purities lead to inconsistent swelling and dimensional changes [8].
• Curing Inconsistency: The intensity of UV light is rarely uniform in a cure box. Research shows that curing in an oxygen-free environment (e.g., glycerine) can significantly improve a retainer’s mechanical properties and dimensional stability compared to standard air curing [8].
The Professional Solution: Statistical Process Control
In a professional manufacturing environment, consistency is not hoped for; it is engineered. This is achieved through Statistical Process Control (SPC), a methodology that uses data-driven analysis of key outputs to calculate process capability statistics. A key metric, the Process Capability Index (Cpk), serves as a quality grade for the manufacturing process itself. A high Cpk value provides mathematical proof that the process is so consistent and centered on the target that it will reliably produce parts meeting tight clinical specifications.
This high level of process capability virtually eliminates the need to inspect every part for accuracy. This advanced approach to quality assurance, which moves from inspecting products to statistically guaranteeing the process, represents the standard of care at dedicated manufacturing facilities like Specialty Appliances but is typically beyond the scope of a clinical office setting.
The Business Case: Managing the True Cost of Quality
The decision to print in-house must be evaluated through the lens of the “Cost of Quality.”
The Cost of Poor Quality (Failure Costs)
These are reactive expenses when controls fail, including appliance remakes, lost chair time, reputational damage, and liability. Real-world examples exist in the FDA’s MAUDE database, which documents patient allergic reactions and device failures due to poor fit from 3D-printed dental appliances [9, 10].
The Cost of Good Quality (Prevention & Appraisal Costs)
These are proactive investments required to prevent failures. This includes capital for validated equipment, recurring costs for materials and calibration, and significant “soft costs” for training, documentation, and validation studies. A critical consideration is the rise of “validated” in-office systems marketed as complete solutions. While these systems can lower the initial barrier, they do not absolve the practice of its responsibilities. The FDA and international standards (like ISO 13485) place the final responsibility for process validation on the legal manufacturer — the entity printing the device [3]. A practice must still perform site-specific validation (IQ, OQ, PQ) for any system in their facility to ensure it produces safe and effective devices, which represents a significant and ongoing cost.
A Balanced View: When Can In-Office 3D Printing Be Appropriate?
This paper’s cautionary tone is not intended as a wholesale rejection of in-office 3D printing. Rather, it is a call for a clear-eyed assessment of its responsibilities. In-office printing can be a safe and effective tool under specific, controlled conditions:
1. For Non-Critical Applications: Printing items that are not used to fabricate patient-contacting devices and do not require high dimensional accuracy, such as initial diagnostic models or visual aids, carries significantly lower risk.
2. With a Full Commitment to Quality: For practices that are willing and able to fully embrace the “Cost of Good Quality.” This means investing not just in the equipment, but in developing a robust Quality Management System, dedicating personnel to training and process control, and performing the rigorous validations outlined in this paper.
The critical distinction is between using a 3D printer as a simple office appliance versus operating it as a medical device manufacturing line. For practices that cannot or do not wish to take on the latter, a partnership model is the most prudent path.
Conclusion & Recommendations
The allure of in-office 3D printing is undeniable, but its implementation requires a profound understanding of the associated responsibilities as a medical device manufacturer. The data is clear: a lack of rigorous process validation exposes patients to cytotoxic materials, legal liability, and practices to significant financial and clinical risk.
It is imperative for clinicians to recognize that 3D printing is not an appliance — it is a manufacturing process. To ensure patient safety and success, every practice must either commit to the substantial investment required to build and maintain a compliant quality system or choose a partner who has already met this standard. By partnering with a dedicated manufacturer that has made these investments — of which Specialty Appliances is an example — clinicians can confidently deliver the highest standard of care, secure in the knowledge that every appliance is backed by a validated, data-driven process that prioritizes the well-being of the patient.
Disclaimer
This publication is intended solely for licensed dental professionals and is provided for informational and educational purposes only. It does not constitute medical, legal, or regulatory advice and should not be relied upon as a substitute for professional judgment or consultation with qualified regulatory experts. The content reflects current knowledge, research, and emerging technologies related to 3D-printed orthodontic appliances as of the date of publication. While every effort has been made to ensure accuracy, no warranty is made, express or implied, regarding the completeness, accuracy, or regulatory compliance of the information presented. Dental professionals are responsible for ensuring that any 3D-printed orthodontic device they design, manufacture, or use complies with applicable federal regulations, including but not limited to the U.S. Food and Drug Administration (FDA) requirements for medical devices under 21 CFR Part 820 (Quality System Regulation), 21 CFR Part 807 (Registration and Listing), and other relevant sections. The authors and publishers expressly disclaim any liability for clinical outcomes, regulatory noncompliance, or other consequences arising from the use or interpretation of this material. References to specific products, manufacturers, or technologies are provided for illustrative purposes only and do not constitute endorsement. All trademarks, product names, and company names are the property of their respective owners.
References
[1] Prakash, J., et al. “Biocompatibility of 3D-Printed Dental Resins: A Systematic Review.” Cureus, 2024.
[2] Cabrol, L., et al. “Effectiveness of postprocessing on 3D printed resin biocompatibility in prosthodontics: A systematic review.” Journal of Prosthetic Dentistry, 2024.
[3] 3DResyns. “Validation of 3D printed medical devices.” 3dresyns.com.
[4] Specialty Appliances. Internal Test Report BCT-0051. Data on file.
[5] Specialty Appliances. Internal Test Report BCT-0066. Data on file.
[6] Specialty Appliances. Internal Test Report 22T-51798. Data on file.
[7] Specialty Appliances. Laboratory Notebook #3, pp. 11-12. Data on file.
[8] Al-Dwairi, Z.N., et al. “Effect of post-processing on the surface, optical, mechanical, and dimensional properties of 3D-printed orthodontic clear retainers.” Clinical Oral Investigations, 2025.
[9] U.S. Food and Drug Administration. MAUDE Adverse Event Report: MDR 16971057.
[10] U.S. Food and Drug Administration. MAUDE Adverse Event Report: MDR 19050179.